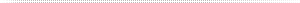Contamination such as humidity, oxygen or microbiological ingress can impact drug stability throughout the product life cycle. To prevent the risks of stability failure of highly moisture sensitive drugs (e.g. dry powder for inhalation), or the risk of biological ingress of parenteral drugs, highly sensitive integrity tests are required. Most test methods are very challenging in regards to time, effort, complexity or the limitation of sensitivity and detection range.

Pfeiffer Vacuum offers a comprehensive portfolio of highly sensitive leak detection and integrity test solutions. The company is benefiting from over 50 years of experience in leak detection, starting with the introduction of the first commercial helium leak detector ASM 4 in 1966. Dedicated for the pharmaceutical industry, Pfeiffer Vacuum offers its innovative AMI Integrity Test System, which does not require any specific tracer gas. Instead, the gas mixture present in the container headspace of the primary packaging is used to perform high sensitivity tests over a large detection range. Suitable for various packaging types such as blisters, plastic & glass bottles, pouches and more, this method is deterministic, non-destructive as well as easy to set up and use.
Leak Sizes and Classifications
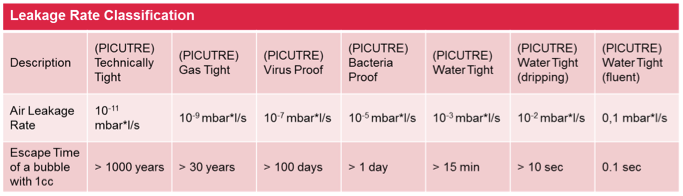
To understand the challenges in regards to integrity testing, it is important to get an understanding for the classification of leaks and the potential effects of different leak sizes. Especially important for the pharmaceutical industry is the criteria for „bacteria proof“ and „virus proof“. Therefore, integrity test methods in the pharmaceutical industry are classified with a “Limit of Detection” index, indicating the smallest leaks that can be detected with a certain test method. An overview is given in the chart below.
Limit of Detection Classification according to USP 1207.1
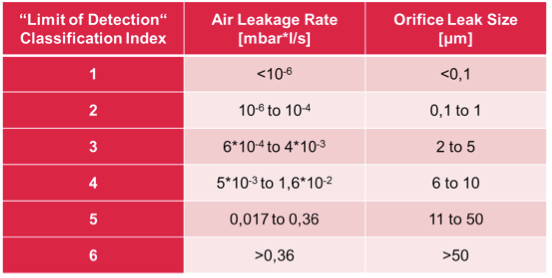
Historically, dye ingress testing has been the container closure integrity test of choice. The detection limit of this method, with a well trained operator, is around 20 μm. Since the test is strictly visual, the detection limit has been experimentally estimated by using orifices of known diameter. As a consequence, tightness criteria in the pharmaceutical industry are usually expressed as an equivalent hole diameter in μm. However, an equivalent hole diameter is not enough to define the tightness criterion of the packaging. Indeed, the flow leak rate only is directly correlated to the quantity of oxygen or moisture you will get inside a leaky packaging.
Helium leak detection is still the most sensitive method for container closure integrity testing. However, some limitations are related to the admittance of the tracer gas. Consequently, attempts have been made to quantitatively test integrity without any specific tracer gas. These methods are e.g. pressure and force decay or optical methods using laser and camera to measure the deformation of the cavities top foil under vacuum. An overview of leak testing methods used for blister integrity testing is shown in the table below:
Equivalent hole diameter (µm)
Highly Sensitive, Quantitative Measurements in Real-Time without Specific Tracer Gases
With the AMI Integrity Test System, Pfeiffer Vacuum has introduced optical emission spectroscopy as a method with lower detection limits compared to any other method that uses gas trapped in the cavity. The packaging to be tested is put into a test chamber which also provides a viewport and mechanical support for the package. The test chamber can thereby be customized according to the product formats and the quantity of products tested simultaneously.
After loading the sample, the chamber is evacuated. At pressures lower than 10-2 mbar, a plasma is ignited and its optical emission analyzed with an optical emission spectrometer. The lowest detectable signal corresponds to an orifice diameter of roughly 0.1 μm (according USP <1207.1>). For further coarse leak tests the AMI sensor technology can be complemented with a second dedicated leak sensor integrated into the same test equipment to extend the upper detection limit up to few mm holes. This gives the AMI the broadest detection range in the market.
The software solutions used in the AMI are compliant with 21 CFR part 11. Optional software solutions are available for a manufacturing execution system. Trend analysis can be implemented in the software for early indication of drift production and packaging equipment.
This deterministic method is easy to set up and to use and yields quantitative as well as highly repeatable results. In addition to the information achieved by a simple GO/NOGO test method, the new method of the AMI allows the detection of drifts in sealing parameters in real time. The loss of valuable pharmaceuticals is prevented and production stops for corrective measures are minimized.
The cycle time depends on the desired detection limit. For a leakage rate of 1.0 · 10-4 mbar·l/s (about 1 μm according <USP 1207.1>), a cycle time of 30 seconds can be expected. Automatic calibration is implemented into the test equipment using certified calibrated leaks. Thereby, operator-independent calibration and test results are provided.
 |
 |
 |
High sensitivity tests over a large detection range are suitable for various packaging types. |
||
Packaging Types and Detection Ranges
The AMI can be used for different types of packaging and sealed objects like medical devices and battery cases. Thereby different sample sizes and detection limits are applicable. In regards to blister packages for example, the AMI can detect holes up to 0.4 μm respectively 2·10-5 mbar l/s (according to <USP 1207.1>). Thereby multiple blisters can be tested simultaneously. About the same detection limit is provided for plastic bottles. Here up to 100 bottles can be tested at the same time. For glass vials the detection limit goes down to even 1/10 μm hole or 1·10-6 mbar l/s leak rate (according to <USP 1207.1>). Also, no sample preparation or storage time is required and the test results will show within about 1 minute.
USPs & Customer Benefits
For more information on Pfeiffer Vacuum please visit www.pfeiffer-vacuum.com.
To read more about Laboratory Applications, please visit www.blowervacuumbestpractices.com/industries/medical.