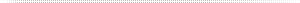To determine which type of vacuum pump will be most suitable for an application, it is important to understand the distillation process, the factors that make distillation work, and the features of the different pump types.
How Does Distillation Work?
Distillation, degassing, drying, filtration, membrane separation, adsorption, and crystallization are all among the separation processes that rely on the differences in the physical properties of substances in a mixture. Distillation relies on the differences in boiling point or in vapor pressure versus temperature characteristics. Heating, evaporation, and condensing are the tools used in distillation that separate the liquid constituents in a liquid mixture.
For separating liquid constituents with differences in boiling points of less than 30 °C, a fractionating column with plates or packing is normally recommended. This provides repeated condensing and re-evaporation of the reflux liquid as it progresses up the column for better separation of the constituents.
Depicted is an example of distillation column set-up for separation processing.
More volatile liquids have lower boiling points or higher vapor pressure versus temperature curves and more readily evaporate. The vapor phase mixture is richer in the more volatile compounds and can then be condensed, contained, and returned for further separation and purification, if necessary. The greater the difference in the volatility of a component from the mixture, the more easily it is separated.
Understanding Volatility
Volatility is the concentration of a substance in a solution (or its mole fraction) in the vapor phase compared to the concentration of the same substance in its liquid phase. Volatility is also a substance’s pure component pressure compared to its total pressure. The relative volatility of two substances is the ratio of their pure component vapor pressures.
Volatility of substance “i” is defined as: Ki = yi/xi. Where,
Ki is the volatility of the i component.
yi is the mole fraction of the i component in the vapor phase.
xi is the mole fraction of the i component in the liquid phase.
(Mole fraction is the ratio of the number of moles of a substance to the total number of moles in a solution.)
The ratio is the same between a substance’s pure component pressure and its total pressure:
Since yiP = xiPvi, where P is the total pressure and Pvi is the pure component vapor pressure: then yi/xi = Pvi/P.
The relative volatility “α” of two substances is: α = K1/K2 = (y1/x1)/(y2/x2) = Pv1/Pv2
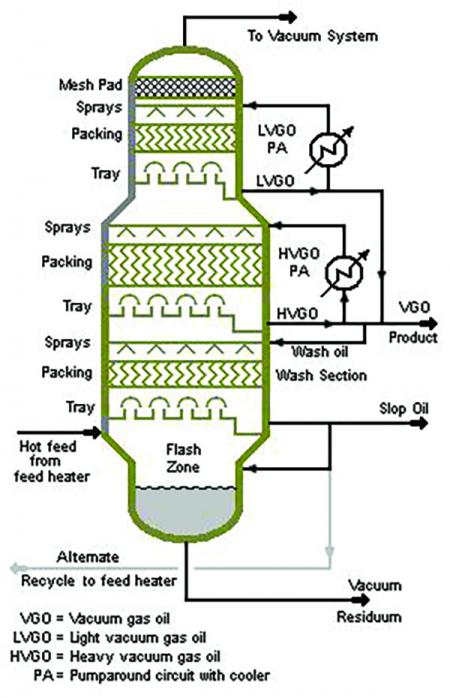
For a simplified binary mixture that behaves as an ideal liquid, a phase diagram at constant pressure can be drawn with the mole fraction of the more volatile component on the horizontal axis and the temperature on the vertical axis.
The lower curve is normally referred to as the “bubble point” where, for a given mole fraction of liquid mixture, the liquid begins to boil at a given temperature. The higher curve is normally referred to as the “dewpoint,” which indicates the different temperatures where the different mole fractions of the vapor would start to condense.
As an example, Figure 1 represents a phase diagram at constant pressure for a well-behaved binary mixture. It shows the mixture will boil at 0.59 Temperature (T) when the more volatile Component 1 represents 0.3 mole fraction of the liquid mixture and will have a saturated vapor “y1” that represents almost 0.75 mole fraction of the entire vapor. This large difference between the vapor and liquid contribution of Component 1 makes it easier to distill off.
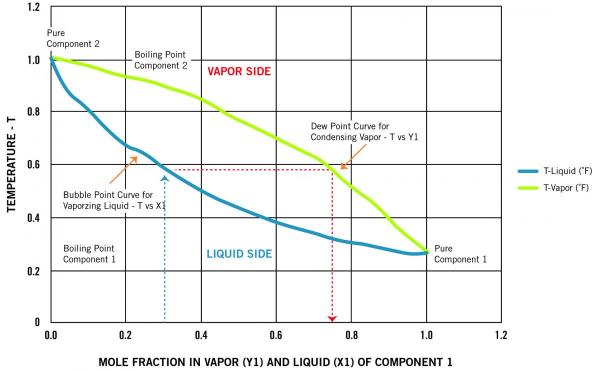
Figure 1.
The Vacuum Distillation Process
For a simplified binary mixture that behaves as an ideal liquid, vacuum distillation provides a convenient and efficient format for the separation at lower temperatures without harmful reactions with other gases such as oxygen.
In some cases, a mixture of two or more liquids at a given mole fraction of constituents will behave as a pure liquid, where the vapor that boils off at a constant temperature has the same mole fraction in the vapor phase as in the liquid phase and no further separation of the constituents occurs. This is known as an “azeotrope.” For example, a mixture of ethanol and water will separate through simple distillation until the mole fraction of ethanol reaches 0.895 and no further change in concentration will occur.
Some azeotropes can be separated by changing the pressure at which distillation occurs. Vacuum distillation can help in some of these cases by providing a pressure variation for shifting the azeotrope to allow for further separation. The ethanol/water azeotrope disappears at distillation pressures below 70 millimeters (mm) HgA. As in all processes, the cost of further separation dictates its feasibility.
Understanding Flow for Proper Vacuum Pump Sizing – Webinar RecordingDownload the slides and watch the recording of the FREE webcast to learn:
|
The Molecular Distillation Process
Molecular distillation is a similar process but occurs at much lower pressures (normally from 0.1 to 0.0005 mm HgA) so that collision of the distillate molecules with the condenser predominate compared to intermolecular collisions.
A thin film distillation process using Wiped Film Stills (WFS) and Evaporators (WFE) provides a convenient method for separating out compounds for the chemical, food, or pharmaceutical sectors that have high boiling points, or high viscosity, or are sensitive to thermal degradation but are readily evaporated at modest temperatures at low pressures.
Preferred Vacuum Pumps
Condensers are used for knocking out most of the condensable vapors. However, for removing the permanent gases, including air leakage and the saturated vapors at the exhaust temperature of the vent condenser, the most common and preferred pumps for simple or fractional vacuum distillation are the liquid ring and dry vacuum pumps. For lower pressure operation, a Rotary Lobe Booster can be connected in series with either of these to provide higher pumping capacity at a lower pressure.
The liquid ring does not require internal lubrication and can run on most liquids such as water, low viscosity oil, or many solvents that are compatible with its materials and its process in terms of vapor pressure and viscosity. It can handle liquid slugs from process upsets or a continuous flow of liquid condensate from a pre-condenser.
In some cases a liquid ring can perform as both a vacuum pump for non-condensables and a direct contact condenser for vapors, increasing its overall pumping capacity. It is one of the most reliable and durable mechanical pumps because of its simplistic design with one rotating shaft assemblage. It is also available in 316 stainless steel for greater corrosion resistance to process effluents.
Shown is a KLRC 300 Kinney liquid ring vacuum pump.
The rotary screw dry pump also does not require internal lubrication and can handle some liquid carryover, but as the name implies, it is better to keep the pump dry for optimum performance.
Knockout pots would normally be recommended to trap out liquid slugs. Since the dry pump contains no liquid within its pumping chamber, it is not limited by the vapor pressure of the liquid and can achieve lower pressures without producing process-contaminated waste products. The dry pump handles condensable vapors by keeping them in the vapor phase at an elevated temperature while traveling from suction to discharge, so they can be condensed out in an after-condenser. The rotary screw dry pump and rotary lobe boosters are also available with optional protective coatings.
Because of the low-pressure requirements for molecular distillation and reduced carryover, multi-stage booster packages utilizing liquid ring, dry rotary screw, or oil sealed rotary piston vacuum pumps as the atmospheric stage are available.
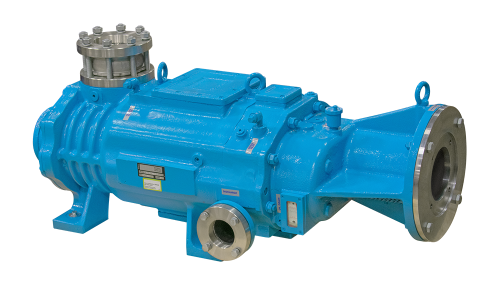
Shown is a Kinney SDV 800 screw-type dry vacuum pump.
Here are advantages and disadvantages of liquid ring and dry pumps:
|
Dry Pump Advantages |
Dry Pump Disadvantages |
|
Lower ultimate pressure and higher capacity at low-pressure end for single-stage pump. |
Higher purchase price. |
|
Lower power consumption. |
Higher complexity affects reliability. |
|
Lower cooling water usage. |
More difficult to disassemble and reassemble on site by end user. |
|
More compact footprint. |
Solvent handling limited by auto-ignition temperature of solvent. |
|
Can pump high vapor pressure solvents. |
Limited liquid ingestion. |
|
Environmentally friendly with less pollution. |
|
|
Liquid Ring Pump Advantages |
Liquid Ring Pump Disadvantages |
|
Can perform as both vacuum pump and direct contact condenser. |
Normally higher operating cost than dry. |
|
Lower purchase price. |
Higher power and cooling water consumption. |
|
Simplicity of rotating parts improves reliability. |
Larger footprint. |
|
Low maintenance. |
Pump performance is limited by vapor pressure of sealant. |
|
Because of pump simplicity, can be readily disassembled and reassembled on site by end user. |
Requires a supply of liquid sealant for makeup or change-out. |
|
Lower operating temperature for thermal sensitive or polymerizable process material. |
Operation normally results in larger amount of hazardous waste. |
|
Liquid sealant allows for handling higher temperature inlet gases/vapors. |
|
|
Can ingest liquid from process or condensater from upstream condenser. |
|
|
Less sensitive to process particulate due to larger clearances. |
|
|
Liquid within pump may act as quench to reduce chance of ignition from sparking. |
|
Liquid Ring Versus Dry Vacuum Pumps
In choosing the best pump for the application, many factors need to be considered concerning the substances that need to be distilled, substances that need to remain in the liquid solution, the pump types themselves, and other aspects of the process:
- Boiling points of substances.
- Vapor pressure versus temperature curves of substances.
- Viscosity and sensitivity to thermal degradation of substances.
- Liquid carryover the pump will need to withstand.
- Pump’s ability to handle liquid slugs from process upsets.
- Need for providing higher pumping capacity at a lower pressure.
- Pump’s level of corrosion resistance.
- Footprint of the pump and complexity of maintenance.
- Pump’s purchase price versus power consumption prices.
- Process cooling water usage and hazardous waste production.
About MD-Kinney
The M-D Pneumatics and Kinney brands have a 100+ year history of providing long-lasting quality products and reliability to the industrial markets, but our most valuable assets reside in our knowledgeable co-workers and MD-Kinney representatives who manufacture, service, and build strong relationships with our customers. For more information, on MD-Kinney brands visit us online to learn more about our history and who we are.
All photos courtesy of MD-Kinney.
To read similar articles on Industrial Vacuum Technology, please visit https://www.blowervacuumbestpractices.com/technology/.